WP5- In silico tissue death and survival
In silico models of perfusion defects and tissue damage
Watch this video (April 2022) by Praneeta Konduri on Modelling tissue damage due to acute ischemic stroke
In this WP, we will examine the effects of both thrombolysis and thrombectomy on tissue perfusion and metabolism. An occlusion in a major artery will result in downstream hypoperfusion; as time progresses, this hypoperfusion will lead to damage to both the vasculature and the surrounding tissue in complex ways, for example through pericyte swelling and death and irreversible blockage of the microvasculature, as well as blood-brain-barrier (BBB) breakdown. Over time, the necrotic core thus grows as a number of processes, also including inflammation, continue to affect the surrounding tissue.
Two ways of restoring perfusion that have been shown to have clinical benefit are thrombolysis and thrombectomy. These are modelled in WP3 and WP4 respectively. However, these do not always result in a restoration of perfusion and healthy tissue functioning, for a number of reasons. These can either due to a failure to restore perfusion, through continued occlusion of a major vessel of multiple occlusions at the microvascular level, or to restoring perfusion to a damaged vasculature and tissue, which can have a deleterious impact on such regions of the brain.
In this WP, we will develop and validate models of blood flow and metabolism related to occlusion and therapy. These will then be used to answer the clinical questions of how reperfusion can be improved and how therapy might be better targeted, by being adapted dependent upon the time post-occlusion in order to gain the maximum benefit from either thrombolysis or thrombectomy. As a result, the full dynamic response to clot formation and removal will be understood. The modelling work and validation experiments will be focussed throughout on the penumbral tissue, i.e. the regions where early and adequate reperfusion has the potential to salvage tissue and where small differences in local perfusion, for example due to micro-emboli, can have large effects on oxygen and glucose supply to tissue and hence final outcome.
In the first part of the WP, the emphasis will be on developing models of the interaction between a clot in a large vessel and the downstream circulation and tissue, with the later emphasis being on the interaction between smaller clots and the microcirculation and tissue. This will then be related to cellular metabolism and tissue damage and death. Through the use of multi-scale techniques, behaviour at the microvascular and cellular level will be characterised at the tissue level, enabling predictions to be made of infarct volume and the response to reperfusion, enabling validation to be performed with clinical patient data.
In the multiscale modelling of the pathophysiology of acute ischemic stroke and its treatment, we identify four main processes (Figure below)
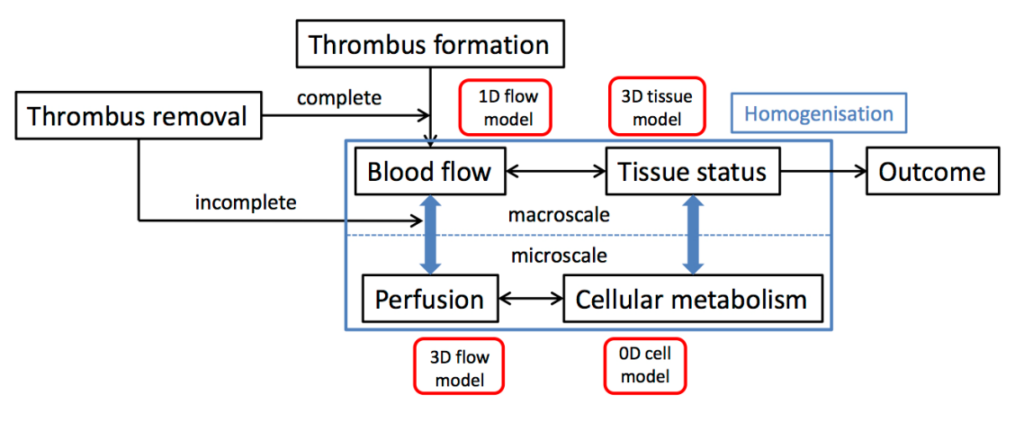
A schematic overview of the multiscale model for acute ischemic stroke and treatment
The impact of vessel occlusion on brain tissue will thus be simulated using a four-component model: 1. thrombus formation, thrombolysis and thrombectomy in WP3 and WP4 respectively; 2. 1-D model of the main vessels, their intra-cerebral branches and collateral connections; 3. downstream microcirculation, represented using homogenization techniques; 4. 3-D biochemical model of cell death in response to ischemia, again using a homogenized approach and parameter values previously validated using clinical data in Oxford. As input this WP uses the patient data from WP2 and incorporates results of detailed modelling of thrombolysis in WP3 and thrombectomy in WP4. Validated models will be delivered to WP6, where the final integration into the in silico clinical trial will be carried out.
Objectives
1. Modelling of microvascular and tissue damage post-occlusion.
2. Multi-scale modelling of the relation of vascular occlusion by thrombi with microvasculature impairment;
3. Validation through data from literature and from imaging and experimental data;
4. Provide high-scale models of tissue damage as input for in silico clinical stroke trials describing tissue damage as a function of time from stroke onset-to-recanalization/reperfusion.
Update September 2020
In WP5, we have been developing new models of blood flow and oxygen transport in the brain. These have been built up from the very smallest vessels (capillaries) to the large supply vessels (arteries) to the brain. Linking all of these models together has been a big challenge, but we can now model blood flowing all the way through the brain. Since blood transports oxygen to the brain cells, we have also been able to model how oxygen is delivered to brain tissue for the first time in such a detailed way. This is important because it means that we can simulate not only the response of the brain to a stroke, i.e. when a large vessel is blocked, but also the response of the brain when a clot is removed, and some very small clots remain in the bloodstream, blocking smaller vessels downstream (which can be a problem if there are lots of these). In parallel with the modelling work, we have been performing animal studies, looking at how these small clots affect both the transport of oxygen to tissue and how it dies in response. In the last year of the project we will be tying all these models together and validating them using both the animal data and clinical data. This will mean that we will have a fully-validated simulator of stroke for the first time that can be used to help in other WP to simulate the response to stroke and its treatment.

A statistically accurate model of the capillary bed in human grey matter
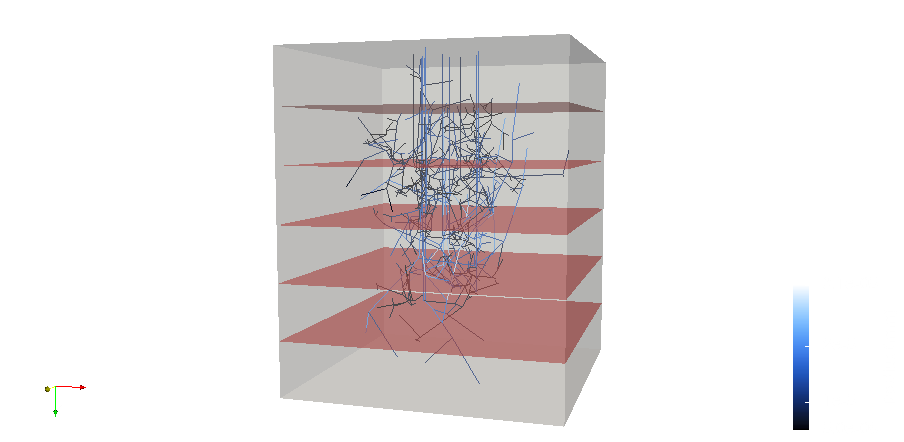
Penetrating vessels descending into a voxel of grey matter. Red layers indicate slices over which permeability was calculated
