WP4- In silico models for thrombectomy
Watch this video (April 2022) for the latest update on In silico modelling of mechanical thrombectomy
Update September 2020:
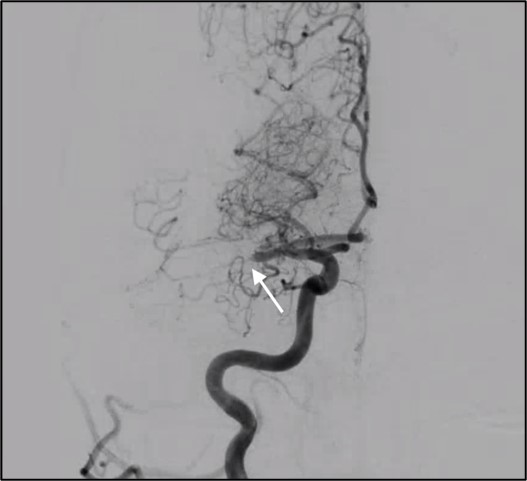
Intra-arterial thrombectomy is a minimally invasive procedure based on stent technology. After the stent-retriever deployment, the thrombus is dislodged by retrieval of the stent.
Patient-specific details of the procedure were collected, and thrombus characteristics were included in the model. Geometry of the stent-retriever was created to replicate the real design of the device.
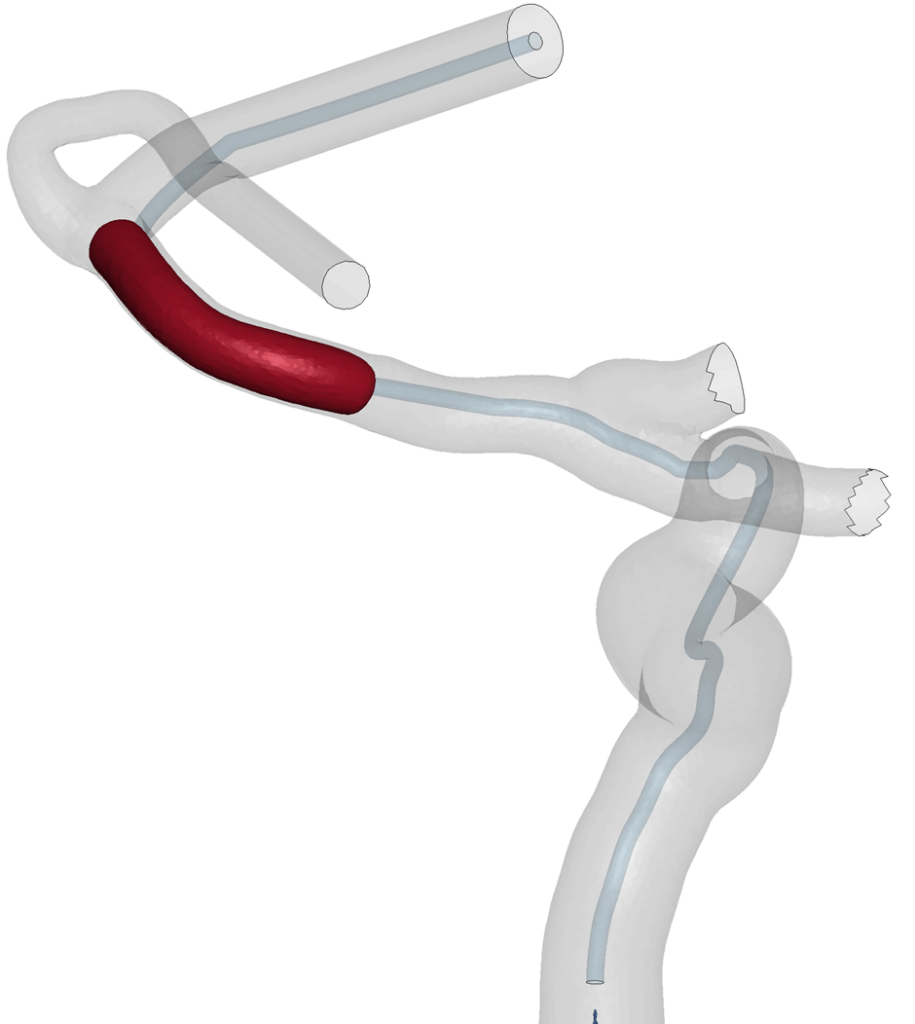
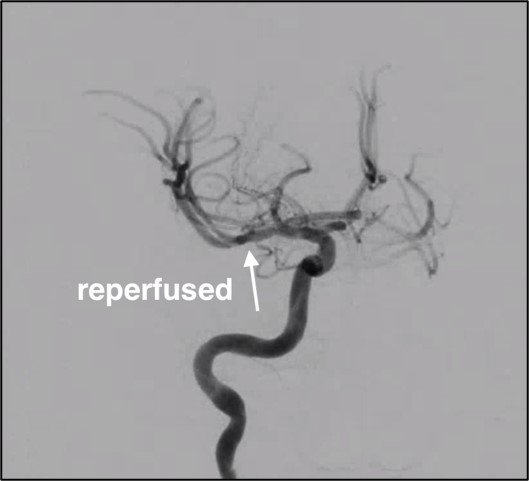
The simulations showed that our models can replicate the thrombus extraction. The proposed workflow is amenable to model the thrombectomy procedure and it could be used to predict the procedure outcomes but also to optimize the procedure itself or the stent-retriever design.
In this WP, a new understanding of the mechanical behavior of clots will be developed and this knowledge will be used to develop novel constitutive laws and fracture models for clots. We will develop a modelling platform in which thrombectomy in patient-specific anatomies is simulated. Advanced modelling approaches that accurately describe the mechanical behavior of thrombectomy devices will be developed. This modelling platform will be used as a design tool to deliver optimized device configurations. Additionally, experiments will be performed to provide a new understanding of the interaction between clots and the vessel wall and this complex adhesive process will be described using novel cohesive zone formulations. Such advances on the mechanics, fracture and adhesion of clots will be incorporated into patient specific finite element models generated from medical images. This WP gets input from WP2 (anatomies, clot locations, size, structure) and closely interacts with WP3 (in silico models for thrombosis and thrombolysis) to capture the potential influence of thrombolysis on the thrombectomy procedure.
The thrombectomy simulations will be used to provide estimations on (1) chance of successful recanalization, (2) chance of thrombus fragmentation, (3) chance of embolus in new territory, (4) estimates of procedure time in patient specific configurations. These outcomes will be collected in libraries that are used in the setup of integrated in silico clinical stroke trials.
Objectives
1. Characterization of clot mechanical behavior, fracture and adhesion and development of new clot constitutive formulations;
2. Mechanical characterization and computational modelling of thrombectomy devices.
3. Development of an in silico modelling platform for the simulation of thrombectomy device performance using anatomically accurate patient specific vessels and clots;
4. In silico optimization of commercially available thrombectomy devices and development of new device concepts.
