WP3- In silico models for thrombosis and thrombolysis
Scroll down for updates!
And watch this video (April 2022) for the latest update on thrombolysis: from experiments to computer modelling
In this work package, we will enhance, tune and validate numerical and mathematical models for thrombosis and thrombolysis. For validation of our models, we will closely collaborate with WP2 (population and morphology models) to obtain information from clinical images and clinical trial data, as well as on clot sizes and structure. Finally, we will gather additional biological data, which will be used for tuning the mathematical models, for parameter settings, and for validation. This closely interacts with WP4 (in silico thrombectomy), providing input for the development of finite element models of thrombolytic clots .
This work is divided in three tasks, each following the main phases of the project, see Figure below.
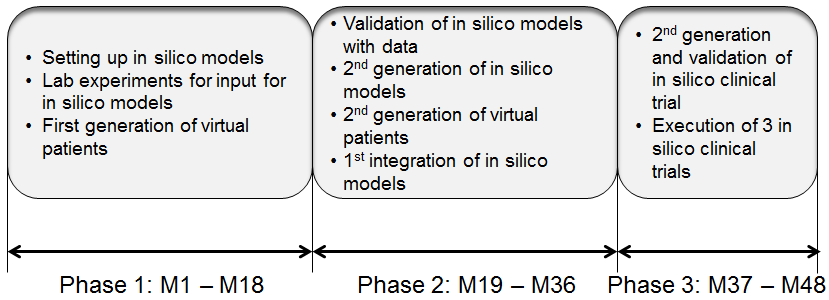
Schematic overview of the timing of INSIST
During the first 18 months, this WP will focus on parameterized models for thrombosis and thrombolysis and deliver first versions of the required combined thrombosis/thrombolysis model to be integrated in WP6 (in silico clinical trials). In the next 18 months, the work continues to further tune and validate these models and make them patient specific, and by using output of WP2, taking into account the type of thrombus (e.g. platelet derived thrombus) and details of the composition, and deliver a further improved combined thrombosis/thrombolysis model to WP6 after three years into the project. During the last year of the project we will continue to further study the details of especially thrombolysis, with focus on the specific needs of our industrial partners IRIS and NEUA and where needed work with WP6 where in this phase of the project several in silico clinical trials will be carried out.
The thrombus formation and thrombolysis models will be developed based on the previous work performed by partners UNIGE and UvA in the FP7 VPH project THROMBUS, by partner LMSU, as well as on new biological findings obtained in this project through in vitro experiments. The numerical models will implement the spatio-temporal transport and reaction processes that lead to thrombus growth or its dissolution. Key blood molecules, including fibrinogen, thrombin, and plasmin, or thrombolytic agents such as alteplase or tenecteplase will be transported by a blood flow model (here a Lattice Boltzmann solver from the Palabos library). These molecules diffuse into the clot region and react to produce new components or destroy existing ones, according to various biological mechanisms, some of them still to be further characterized in the planned experiments. The central questions we will address are (i) patient-specific variations of, e.g. levels of TAFIa and thrombi permeability, (2) the working of alteplase and tenecteplase for different patient specific variations, such as thrombus composition, (3) the working mechanisms of alteplase and ADAMTS13 and its interaction with elevated TAFIa for different patient specific variations, (4) if exporting effect of ADAMTS13 on the presence of von Willebrand factor in thrombi allows a fast restoration of occluded arteries.
Objectives
1. Further development of patient-specific models of thrombosis and thrombolysis;
2. Obtaining in vivo and in vitro biological data related to thrombosis and thrombolysis;
3. Combining thrombosis and thrombolysis models into a sufficiently fast model that predicts i.a. time to recanalization, changes in clot properties (mechanical), and erosion of micro-emboli;
4. Validation of individual models and combined model.
Update September 2020
WP3 has developed three numerical models, with different purposes:
(1) a 1-D macroscopic thrombolysis model aimed to provide calibration and sensitivity analysis.
(2) A 0-D macroscopic model of combined thrombosis and thrombolysis able to describe and understand in-vitro experiments.
(3) A 3-D mesoscopic, parallel HPC model, simulating the thrombolysis in patient geometries and heterogeneous clots. This is the candidate model for the in-silico trials in WP6.
Here is a video from our current simulations for the 3D mesoscopic thrombolysis
(1) a full thrombosis-thrombolysis endogeneous in-vitro experiment giving the time evolution of the thrombus.
(2) A detailed analysis of the histology of the patients clots, showing the variation of composition and spatial heterogeneity (also discussed in WP2).
(3) An analysis of fibrin pure thrombis to determine the fiber diameters as a function of the fibrinogen concentration. (4) the flow of a contrast agent in a thrombus subject to a pressure gradient.
Validations of the numerical models have been conducted against literature data and patient data for porosity and permeability values. Lysis time between the 1D macroscopic model and the 3D mesoscopic mode have been compared. Compatibility with clinical lysis times have also been verified. The 0-D model of thrombosis-thrombolysis has been validated and calibrated from in-vitro observations.
A sensitivity analysis was performed with the 1D model, identifying the most important quantities that affect lysis (concentrations of various molecules, presence of collaterals, and clot heterogeneity). In conclusion, from a modelling point of view, the achievements are on track, but the validation remains a challenge. First, in-vitro and in-vivo data that can be compared with numerical simulations are not always available or easy to produced. Second, the lysis process is still insufficiently understood to describe all relevant situations and have a universal model.
